[This is a fast-moving and controversial topic, so if you’re reading this, you may disagree with what I say, or I may be wrong. Please feel free to read the sources linked throughout my post. If in doubt, please consult with your doctor. Also, I’m writing this as much for myself, to process all the things that I’ve been hearing and reading, so this may or may not address your specific case. #notmedicaladvice]
By the time you read this, if all goes well, I will have received my first vaccine dose. I’ll be getting the AstraZeneca-made vaccine, for a bunch of reasons, perhaps best summed up by this quote from our Prime Minister:
There’s a bunch to unpack here. In order for a vaccine to be offered to anyone, it needs to go through a number of steps, shown in this handy chart from UNC Healthcare:
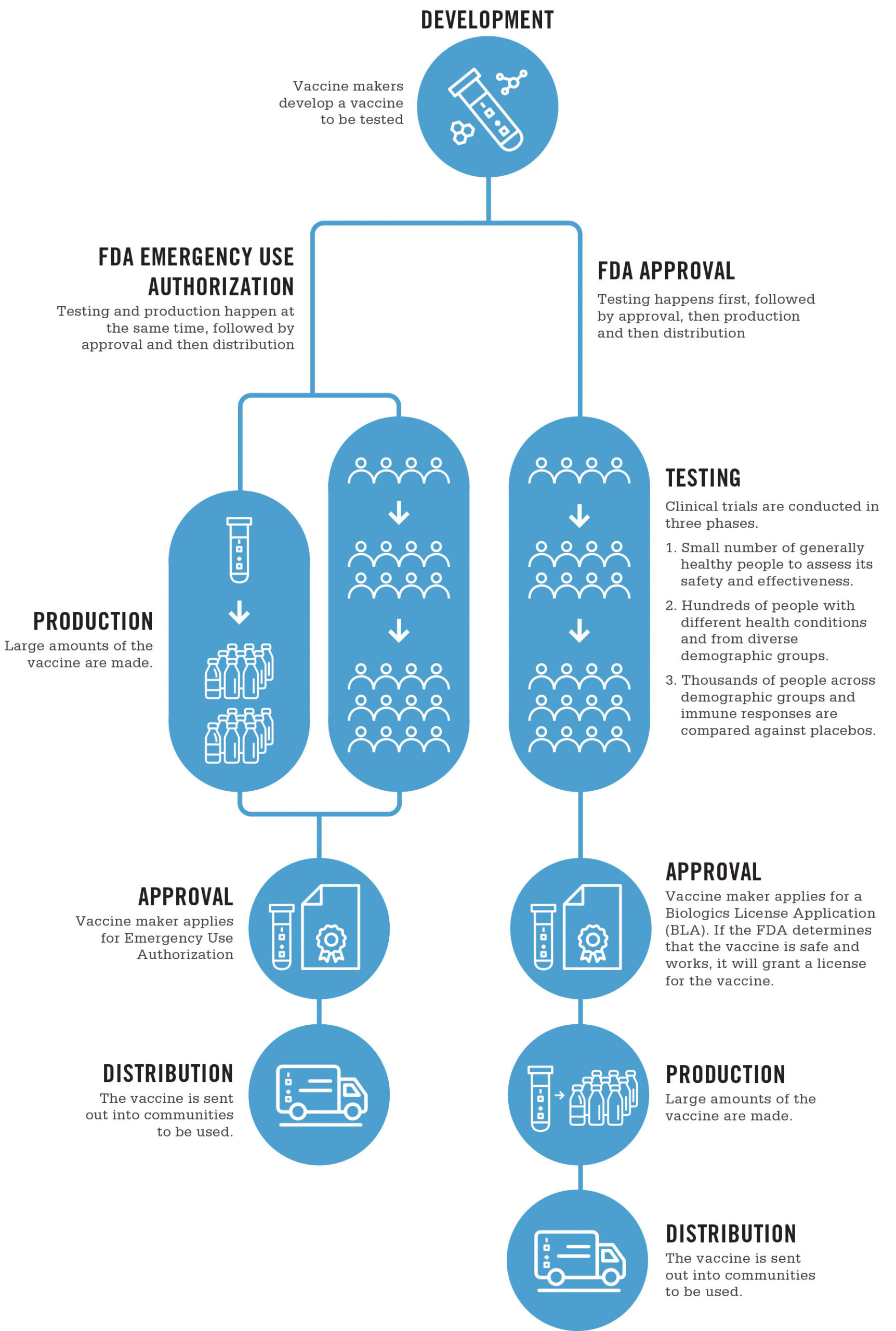
1) The initial R&D of the vaccine, including the conceptualization, and very likely in vitro (cell culture) tests and in vivo (animal) tests, both to show safety and effectiveness
2) Three phases of increasingly large clinical trials, to test for safety & effectiveness
3) Formal approval
The above process is the one for the U.S. FDA, but other jurisdictions will have similar processes. In Canada, the National Advisory Committee on Immunization (NACI) does a review of the evidence, and makes an approval decision. As vaccines can have risks as well as benefits, the NACI may approve vaccines for certain demographics, and not others. A common example of this might be restricting approval to adults 18 and over, due the difficulties and ethical restrictions of testing on children. Indeed, the current statement on the AstraZeneca vaccine includes such a statement:
“The AstraZeneca COVID-19 vaccine is authorized for use in Canada for adults 18 years of age and over. Health Canada has determined that it is a safe and effective vaccine.”
The availability of multiple approved vaccines has led to comparisons of the four vaccines currently approved for use in Canada: Johnson & Johnson, AstraZeneca, Pfizer, and Moderna
This being a fast-moving topic, affecting millions (really, billions) of people, science news is being reported on a daily basis in the popular press, which has a number of effects:
Because the topic is fast-moving, there is a lot of news, not all of it checked to normal standards of scientific rigor.
Because the topic is affecting millions of people, we see effects that we might not otherwise see in small populations. For example, of the approximately 9.5 million vaccine doses administered in Canada to date, there have been 3738 ‘adverse effects’ reported, with 529 of those being deemed ‘serious’, or about 55.5 per million. (For a breakdown by demographics, click here.)
(Apologies for the formatting below, but WordPress is tricky. You may want to rotate your phone to read the table in landscape. The full description of each of the columns is available here, and the names of the columns appears before the abbreviations below.)
Here, you can see a summary of the adverse effects seen in Canada from COVID vaccines so far, as defined here. (Rotate your phone to landscape if the table does not display properly.)
Number of adverse event reports by vaccine name up to and including April 16, 2021 (n=3,738) Vaccine name Non-serious reports Serious reports Total reports Total number of doses administered Total non-serious report rate* Total serious report rate* Total report rate*
Non-S Ser Total Total Rate R(ser) R(non-ser)
Pfizer-BioNTech 1,762 395 2,157 7,183,048 24.53 5.50 30.03
Moderna 1,311 83 1,394 1,843,805 71.10 4.50 75.60
COVISHIELD 124 36 160 491,171 25.25 7.33 32.58
AstraZeneca 11 9 20 615,582 1.79 1.46 3.25
Unknown 1 6 7 N/A NaN NaN NaN
* Per 100,000 doses administered.
(‘COVISHIELD’ refers to the AstraZeneca vaccine, under a slightly different brand name.)
Overall, between all the vaccines administered, there have been:
(From the page, and the recommendations for on-site supervision immediately following vaccination[1], my guess is that deaths associated with vaccination are generally caused by anaphylaxis, but I don’t have good data on that.)
(Please note that this number of 19 per ~9.5 million may go up or down, but as it stands, it’s about at 2 per million, or 1/4 as dangerous as being a pedestrian, or 1/13th as dangerous as driving a car for a year. (2017 data))
2017
https://tc.canada.ca/en/canadian-motor-vehicle-traffic-collision-statistics-2017
Drivers: 985 (26/1e6) Passengers: 311 (8.5/1e6) Pedestrians: 284 (7.7/1e6)
Canada Population: 36,708,083 (approximate)
https://www150.statcan.gc.ca/n1/pub/12-581-x/2018000/pop-eng.htm
(Please also note that all of these vaccines seem to have similar rates of serious and non-serious side effects.)
The item at the top of the news at present is that there are currently specific questions about blood clots and the AstraZeneca vaccine. Health Canada performed a review, and determined:
”
Health Canada’s review of the available information concluded that a link between the use of AstraZeneca COVID-19 Vaccine and COVISHIELD and the risk of these blood clots with low platelets is possible. The risk of these events is very rare, and the overall benefits of the vaccine in protecting Canadians from COVID-19 continue to outweigh its potential risks.
Health Canada did not identify risk factors, such as age or gender, for these very rare events, and is not restricting the use of the vaccine at this time.
A potential mechanism for the combination of blood clots with low platelets is the triggering of an immune response by the vaccine, leading to a condition similar to that seen sometimes in patients treated with the blood thinner medication heparin.
”
(You can see the timeline of updates here. You can see the current ‘product details’ here.)
In the UK, this incidence seemed to be:
”
The potential risk of blood clots with low platelets is very rare. Based on their vaccination rate as of March 31, 2021, the United Kingdom Medicines and Healthcare Products Regulatory Agency estimated the overall risk of these blood clots to be approximately 4 people in a million who receive the vaccine. Reported cases of these adverse events have been seen after the first dose, usually within the first 14 days after immunization.
”
While the overall population risk seems low, when people have options, they will move to optimize their decisions with whatever information they have available, especially when there may or may not be demographic effects on these issues. At its worst, this leads to ‘vaccine shopping’, exacerbating outbreaks, but at its best, it involves people making educated decisions about their personal risks and benefits from taking a particular vaccine. Indeed, from the NACI April 23rd statement:
“At this time and based on current evidence, NACI recommends that the AstraZeneca COVID-19 vaccine may be offered to individuals 30 years of age and older without contraindications, if the individual does not wish to wait for an mRNA vaccine and the benefits outweigh the risk.”
This represents the fact that individuals between the ages of 30 and 40 are at reduced risk for COVID (compared to older individuals), and they may be at the same or increased risk for these blood clots.
There has been speculation that this is auto-immune linked, but the current (not yet published) research has not found (or ruled out) a link yet. (Numbers are still very small, and this is a tricky determination to make.)
However, if you know that you are more susceptible to auto-immune issues (especially those with high estrogen levels), you might want to consult with your doctor, or wait if it remains safe for you to so, while the science is worked out. Ultimately, only you (with your doctor) can make this determination.
However:
Overall, the title of this post still stands. There are a small number of rare side effects associated with these vaccines (mainly PEG allergic reactions for Pfizer & Moderna, and blood clots for AstraZeneca), both of which are detectable and generally treatable. I’m planning to get my shot tomorrow morning, and I believe that the vast majority should also, as soon as they can.
Stay safe.
-Nayrb 🙂
[1] “The Pfizer-BioNTech COVID-19 vaccine is contraindicated in:
– Individuals who have ever had a severe allergic reaction (i.e. anaphylaxis) to a previous dose of an mRNA vaccine or to any of its components (including polyethylene glycol (PEG) and/or polysorbate) or its container, should not get either mRNA COVID-19 vaccine. PEG can rarely cause allergic reactions and is found in products such as medications, bowel preparation products for colonoscopy, laxatives, cough syrups, cosmetics, skin creams, medical products used on the skin and during operations, toothpaste, contact lenses and contact lens solution. PEG also can be found in foods or drinks but is not known to cause allergic reactions from foods or drinks.
– Vaccination should be deferred in symptomatic individuals with confirmed or suspected SARS-CoV-2 infection, or those with symptoms of COVID-19.
– As a precautionary measure and in light of the need to be able to monitor for COVID-19 vaccine adverse events without potential confounding from symptoms of COVID-19 or other co-existing illness, it would be prudent to wait for all symptoms of acute illness to completely resolve.
– Individuals who have received another vaccine (not a COVID-19 vaccine) in the past 14 days.
– Individuals under the age of 16: The safety and efficacy in children under 16 years of age have not yet been established. The manufacturer plans to conduct clinical trials in children.
Considerations for other patient groups
– Guidance for special populations, including for example breastfeeding or pregnant individuals, individuals with allergies, individuals with autoimmune conditions, or individuals who are immunocompromised due to disease or treatment, is available in the Vaccination Recommendations for Special Populations guidance document.
Precautions during vaccination should be taken for:
– Patients who have a bleeding problem, bruise easily or use a blood-thinning medicine should receive the vaccine. Individuals receiving long-term anticoagulation with either warfarin or heparin are not considered to be at higher risk of bleeding complications following immunization and may be safely immunized through the intramuscular route as recommended, without discontinuation of their anticoagulation therapy.
– There is some evidence to suggest that instramuscular administration may be safer when given with a small gauge needle (23 gauge or smaller) and when firm pressure is applied to the injection site for 5 to 10 minutes
– Individuals with a history of severe allergic reactions (i.e. anaphylaxis) not related to vaccines or injectable medications—such as allergies to food, pet, venom, environmental, or latex, etc. should be offered the COVID-19 vaccines.
– An extended period of observation post-vaccination of 30 minutes is recommended for these groups
– For more detailed recommendations on people with allergies, please consult the Vaccination Recommendations for Special Populations guidance document.
” https://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/vaccine/COVID-19_pfizer_vaccine_administration.pdf